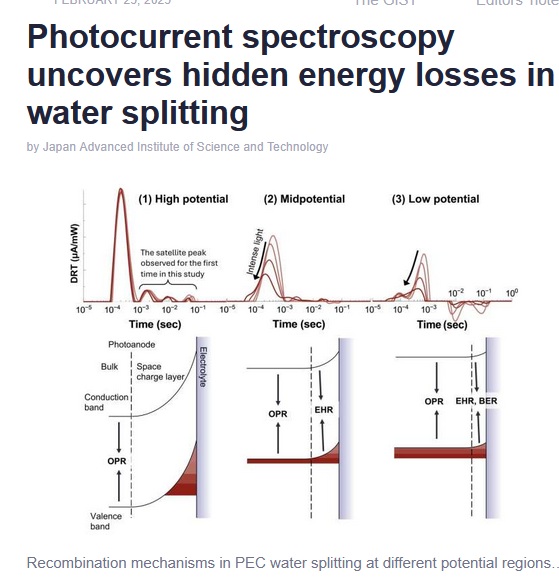
From an article on phys.org, researchers are using new spectroscopy tools to learn about electrons in splitting water into hydrogen and oxygen. They “split water” to generate hydrogen for clean fuels. The newer process to to split water of photoelectrochemical (PEC) is discussed.
That PEC process is one that can scale up better than the old high school chemistry class method of electrodes in distilled water with a catalyst (an acid perhaps)
[may not be accurate here, I have been out of school for a while]
In any event even the new more scalable designs are energy intensive. Part of that is just the chemistry in the H2O bonds.
What is new here is the scientists,have developed a way to see electron movement in detail, revealing previously inseparable processes.
From the article “However, the process in the photoanode suffers from inefficiencies due to electrons and holes recombining before they can complete the reaction. Understanding these losses is essential to improving the technology.”
They say they have found a way to pinpoint the rate-limiting step in water splitting. If you are like me, and you took reaction kinetics or physical chemistry, this is a key to understanding how reactions progress, the rate of reaction, and possibly help optimize a reaction rate [how fast and what energy it takes to create the hydrogen.]
So WIN WIN as we say in P-CHEM!
Here is a link to the article.
https://phys.org/news/2025-02-photocurrent-spectroscopy-uncovers-hidden-energy.html
Leave a Reply